NRX-100/NRX-101
Our Science
Targeting the brain’s NMDA receptor to lessen suicidal ideation
If you know five people with bipolar disorder, on average one will succumb to suicide. After centuries of mystery about the mechanism of bipolar disorder, major breakthroughs in unlocking the mystery began with Professor Daniel Javitt’s 1987 discovery that the NMDA receptor of the brain is the key to maintaining control over the rate at which thoughts are formed and the manner in which brain cells connect to one another.
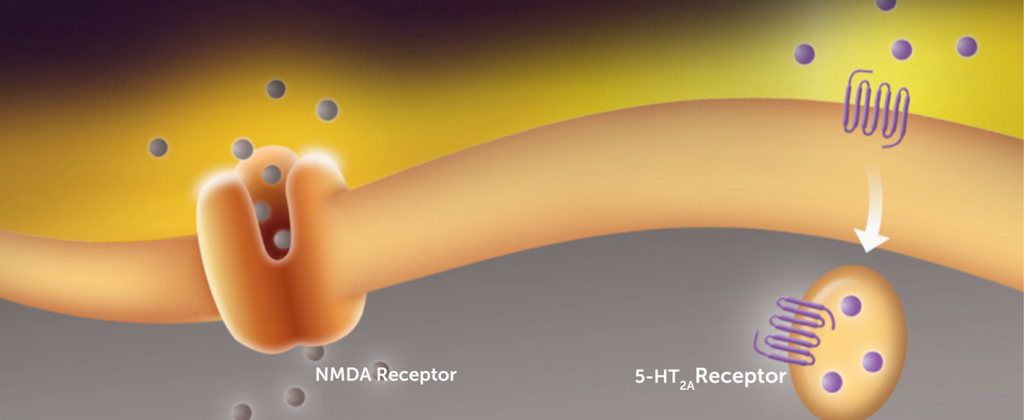
NRX-101’s proprietary dual-mechanism of action targets the NMDA and 5-HT2a receptors – two key receptors in the brain.
Javitt’s work began with an attempt to explain the mechanism of drug-induced psychosis, a condition where people’s thoughts race out of control and hallucinations are common. Over the subsequent 25 years, it has become apparent that the NMDA receptor also has a major effect on depression and, particularly, thoughts of suicide. NRx is on a mission to bring hope to people who suffer from suicidal depression and PTSD through a new generation of NMDA-modulating medicines.
D-cycloserine, patented by Javitt as an antidepressant and antisuicidal drug, is approved worldwide as an anti-infective, primarily used for the treatment of tuberculosis. Although its anti-infective activity is based on its ability to disrupt bacterial cell walls, it also acts as an NMDA antagonist when used at high doses (> 500 mg). By targeting the NMDA receptor and modulating NMDA activity, D-cycloserine seems to foster a normal pace of thought generation. Multiple exploratory clinical studies have demonstrated that administration of D-cycloserine can trigger an antidepressant effect, as well as maintain a reduced level of suicidality.

NRX-100/NRX-101: We are studying Suicidal Treatment Resistant Bipolar Depression
NRX-101 is a fixed-dose combination of D-Cycloserine and Lurasidone. It is the first drug in FDA trials for suicidal bipolar depression.
Clinical trials for
NRX-100/NRX-101
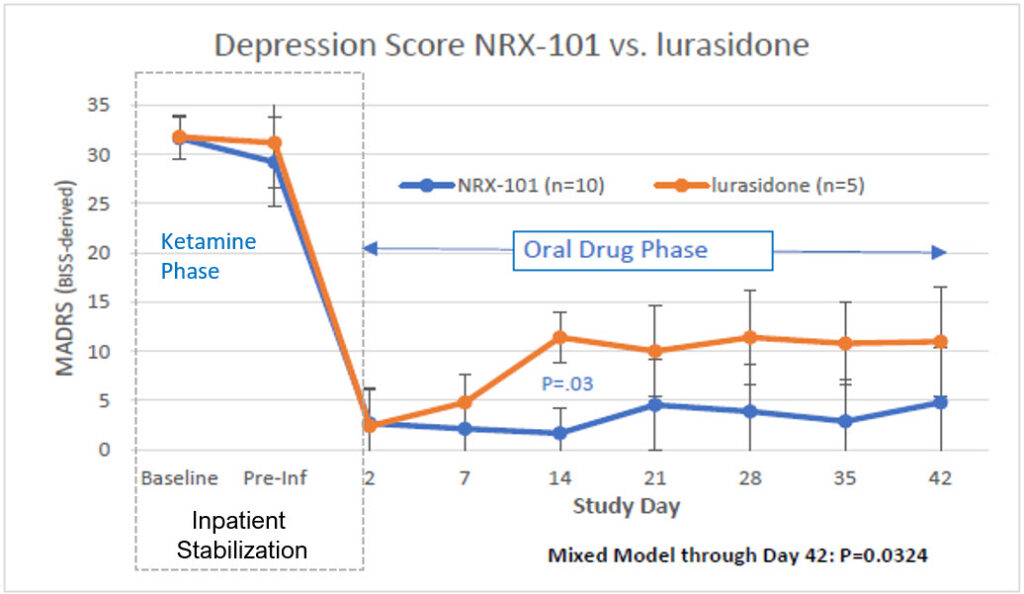
NRx is currently investigating our approach in FDA clinical trials. Results from our STABIL-B phase 2 study were presented at the American College of Neuropsychopharmacology in December 2018, and the FDA granted breakthrough therapy designation (BTD) to NRX-101 based on this study.

Clinical Data
Results from NRx Pharma’s STABIL-B trial, a phase 2 study were presented at the American College of Neuropsychopharmacology in December 2018. The FDA granted breakthrough therapy designation (BTD) to NRX-101 based on this study.
A full, peer-reviewed report of the STABIL-B Trial is published in the International Journal of Bipolar Disorders and can be found here.